Prothena is pioneering protein dysregulation science to advance novel medicines for diseases caused by misfolded proteins, including neurodegenerative and rare peripheral amyloid diseases.

Results from the Phase 3 VITAL clinical trial of birtamimab + standard of care (SoC) versus placebo + SoC in treatment-naïve newly diagnosed patients with AL amyloidosis have been published in Blood. The manuscript includes data on the potential survival benefit with birtamimab observed in a post hoc analysis of patients with Mayo Stage IV disease, who are at high risk of early mortality
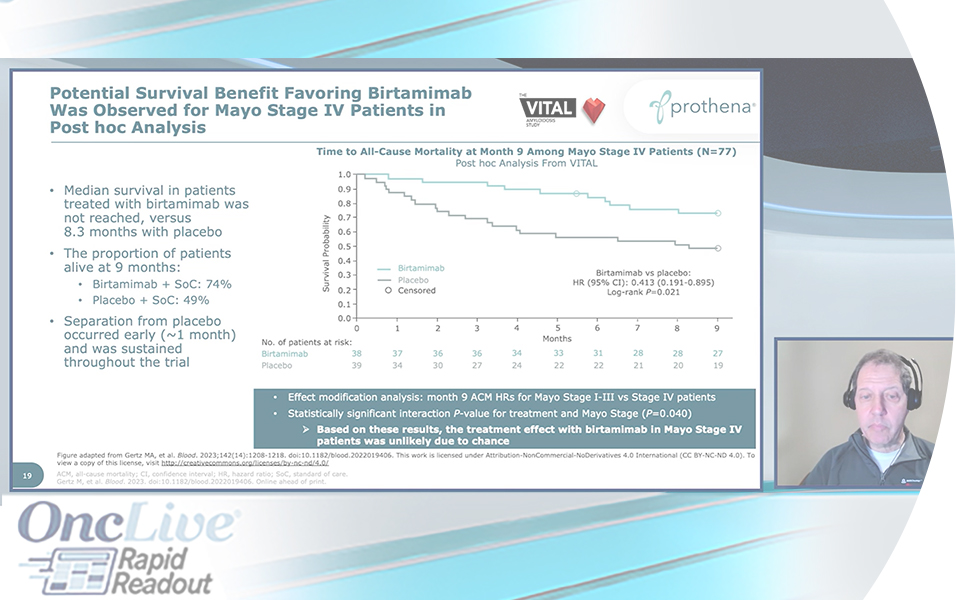
Want to learn more about birtamimab and the Phase 3 VITAL trial results? Check out corresponding author, Dr. Morie Gertz, summarizing the VITAL manuscript in this Rapid Readout video on OncLive®

Prothena’s Phase 3, global confirmatory clinical trial will evaluate birtamimab versus placebo (both with standard-of-care chemotherapy) by assessing time to all-cause mortality in newly diagnosed patients with Stage IV AL amyloidosis; this clinical trial is open for enrollment

Check out the great resources featured on The Amyloidosis Channel on VJHemOncTM. Hear from experts in the field on the latest advances in amyloidosis. The channel includes interviews, roundtable discussions, podcasts, feature articles, and e-learning. Prothena is proud to be a Gold Supporter of The Amyloidosis Channel

This 2-minute video provides an overview of the mechanism of action of birtamimab. Birtamimab is an investigational monoclonal antibody designed to selectively target and clear amyloid deposits in the body

PRX012 is a next-generation, subcutaneously delivered anti-Aβ monoclonal antibody under investigation for the treatment of Alzheimer’s disease. The Phase 1 ASCENT-1 and ASCENT-2 clinical trials with PRX012 are ongoing
